Ground-Water Dating to Assess Aquifer Susceptibility
abstract
Introduction
The U.S. Geological Survey (USGS), in cooperation with the Virginia Department of Health (VDH), has developed the Virginia Aquifer Susceptibility (VAS) Study in support of the Commonwealth’s Source Water Assessment Program (SWAP). The VAS study will use atmospheric tracers (such as chlorofluorocarbons, tritium, and carbon-14) that are commonly present in ground water to determine “apparent age,” which is the time since the water containing these tracers was isolated from the atmosphere. Ground-water apparent age provides information about the flow of water within an aquifer and the susceptibility of a water supply to near-surface contamination. A young apparent age (< 50 years) indicates greater susceptibility to near-surface contamination since relatively little time has passed to allow for attenuation of contaminants in the subsurface, and because many regulated chemicals have been introduced into the environment in large quantities since the mid 1940s, following World War II.
The Safe Drinking Water Act Amendments of 1996 were enacted to ensure that the United States has safe drinking water and that communities are prepared to address water-supply contamination where it occurs. The U.S. Environmental Protection Agency is charged with implementing the Amendments and has mandated that each state must develop a SWAP to identify source areas of public drinking water, assess the susceptibility of public supplies to contamination, and make this information available to citizens. The VDH, Office of Water Programs is coordinating the SWAP for the Commonwealth of Virginia.
Ground-Water Dating Techniques
The water that recharges an aquifer carries the chemical signature of the atmosphere from which it was derived. Atmospheric concentrations of such constituents as chlorofluorocarbons (CFCs) and tritium (3H) have changed over time. Because it can be assumed that infiltrating water is in equilibrium with the atmosphere, the concentrations of these constituents in ground water reflect their atmospheric concentrations at the time the water was isolated from the atmosphere.
The environmental tracers and isotopes present in a ground-water sample were derived from the atmosphere at the time of recharge. Sample collection and analytical methods must minimize or eliminate contact of the sample water with the atmosphere at the time the sample is collected. The sampling and analytical methods for the four techniques used to determine the age of ground water are briefly described below in table 1.
Table 1. Sampling
and analytical methods for ground-water dating techniques
|
|
|
|
|
Chlorofluorocarbons (CFCs) |
Samples are flame-sealed into borosilicate-glass ampules using a special apparatus that excludes contact with the atmosphere. |
Purge-and-trap gas chromatography procedure with electron capture detector |
|
Tritium (3H) |
Samples are collected in 500-mL glass bottles with polycone seals. |
Enriched electrolytically and analyzed by liquid scintillation counting |
|
Tritium/Helium-3 (3H/3He) |
Tritium: Samples are collected in 500-mL glass bottles with polycone seals. |
Mass spectrometry by the helium ingrowth method |
|
Helium: Samples are collected in special copper tubes fitted with stainless steel pinch-off clamps. The discharge end of the tubes is fitted with an overpressure valve to prevent gas bubble formation in the ground-water sample |
Mass spectrometry |
|
|
Carbon-14 (14C) |
Samples are collected in coated-glass bottles fitted with Teflon septum. Sample water is filtered to eliminate contamination from particulates containing carbonate minerals. Establishing a closed path from the water source minimizes contact with the atmosphere. Actual sample volume is dependent upon the pH and alkalinity of the water. |
Accelerator mass spectrometry |
Chlorofluorocarbons
CFCs are stable synthetic organic compounds that were first manufactured in the 1930s for use as refrigerants, aerosol propellants, cleaning agents, solvents, and blowing agents in the production of foam rubber and plastics (Busenberg and Plummer, 1992). CFCs are eventually released to the atmosphere and hydrosphere, and depletion of the Earth's ozone layer has been in part attributed to the rapid atmospheric accumulation of these compounds (Molina and Rowland, 1974). The method developed by Busenberg and Plummer (1992) uses CFCs as dating tools--trichlorofluoromethane (CCl2F, CFC-11, Freon 11), dichlorodifluoromethane (CCl2F2, CFC-12, Freon 12), and trichlorotrifluoroethane (C2Cl3F3, CFC-113). CFC-11 and CFC-12 make up 77 percent of total global production of CFCs (Derra, 1990). CFC-11, CFC-12, and CFC-113 have relatively long atmospheric lifetimes, 45 ± 7 years, 87 ± 17 years, and 100 ± 32 years, respectively (Volk and others, 1997), and documented atmospheric mixing ratios (fig. 1). The concentration of CFCs in ground water is a function of the atmospheric CFC concentration at the time of recharge, recharge temperature, and the chemical and physical processes affecting the solubility of the CFCs along the ground-water flowpath (Plummer and Busenberg, in press; Cook and others, 1995).
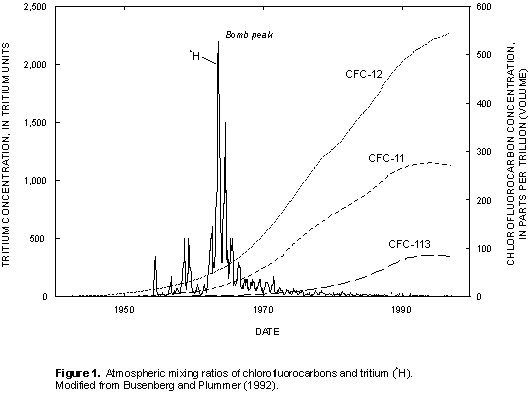
Atmospheric partial pressures of CFCs are determined by Henry's law from the recharge temperature estimated from dissolved gases in the sample (nitrogen/argon ratio) and measured concentrations of CFCs in the ground-water samples. These calculated partial pressures are compared with the atmospheric mixing ratios of CFCs (fig. 1) to determine the CFC-modeled recharge date, which equates to the time that the water was isolated from the atmosphere in the unsaturated zone. Plummer and Busenberg (in press) state that this "clock" is set between the seasonal high and low water table. The apparent age is the difference between the date of sample collection and the estimated date the water entered the ground-water system.
Tritium
Tritium (3H) is the radioactive isotope of hydrogen; it has a half-life of 12.43 years (International Atomic Energy Agency, 1981) and is an excellent indicator of ground water recharged since 1952 (Clark and Fritz, 1997). 3H is released into the atmosphere by cosmic ray spallation, but the principal source is the atmospheric testing of thermonuclear weapons. The standard unit of measure for 3H is a tritium unit (TU), for which one TU is equivalent to one 3H atom per 1018 atoms of hydrogen or, in terms of radioactivity, 3.2 picocuries per liter (Clark and Fritz, 1997). Tritium content in precipitation closely mimics nuclear testing during the 1950s and early 1960s; a maximum concentration occurred in 1963, commonly referred to as the "bomb peak" (fig. 1). Atmospheric concentrations have gradually declined since 1963, and present-day ground water typically contains from <1 to 10 TU, seldom exceeding 50 TU (Clark and Fritz, 1997).
Tritium/Helium-3
Both tritium (3H) and helium (3He) are relatively inert gases. Multiple natural sources of 3He include the Earth's mantle and atmosphere, fluid inclusions within rocks, and excess air entrained in ground water during recharge (Schlosser, 1992). The 3H/3He ground-water dating method is based on the radioactive decay of 3H to 3He. This method separates 3He derived from 3H (tritogenic 3He) from 3He derived from natural sources. Apparent age estimates using the 3H/3He method can be extremely accurate (within months) for ground water containing high 3H concentrations. Unlike the CFCs dating method, the 3H/3He technique can be used at sites that are contaminated with organic compounds.
Carbon-14
Carbon-14 (14C) is continually produced in the atmosphere through cosmic ray bombardment of nitrogen nuclei (Bradley, 1985), and was also introduced into the atmosphere during nuclear weapons testing. With a half-life of 5,715 years, 14C is useful for dating ground water that is between 1,000 and 30,000 years old (Coplen, 1993). Diffusion and geochemical reactions within an aquifer, however, can alter the 14C concentration. A geochemical reaction path model (Plummer and others, 1983; 1991; 1994) is used to account for dilution and addition of carbon along ground-water flowpaths, which improves the accuracy of age determinations.
Virginia's Regional Aquifer Systems
The apparent age of a water sample reflects the integration of natural and anthropogenic elements. Ground-water flow to wells is controlled by factors that are unique to the diverse hydrogeologic settings across Virginia. Water can move through pore spaces in unconsolidated sediment, along fractures and bedding planes in bedrock, or through the solution channels associated with karst areas. Changes in climate, land use, or water use can further influence how ground water flows to a well. As water moves through an aquifer, chemical, biological, and physical processes can affect the composition and concentrations of contaminants that may be transported from the surface. The potential for contaminants in ground water to degrade by chemical, biological, and physical processes along the ground-water pathway increases with the elapsed time between ground-water recharge and discharge. Since aquifer type, as well as ground-water travel times, affects the transport and behavior of contaminants in ground water, the regionalization of apparent ages by hydrogeologic province can establish a basis for the determination of the susceptibility of ground water to near-surface contamination.
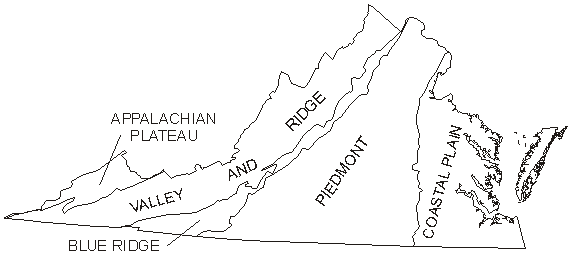
Virginia's aquifers can be grouped into five categories based on the State's physiographic provinces: the Coastal Plain, Piedmont, Blue Ridge, Valley and Ridge, and Appalachian Plateau (fig. 2). Because ground-water flow and interaction with surface water are dependent on physiographic and climatic setting (Winter and others, 1998), the susceptibility to contamination of each of these regional aquifer systems must be evaluated individually.
Figure 2. Physiographic
provinces of Virginia.
Coastal Plain
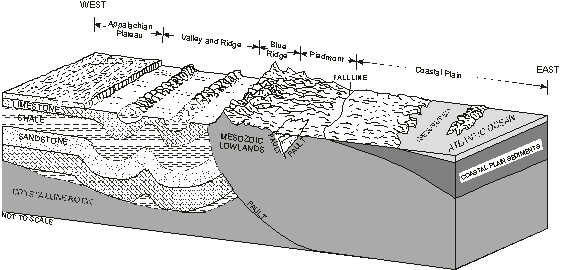
Figure 3. Representative
geologic section across Virginia (modified from Trapp and Horn, 1997).
Piedmont
Blue Ridge
Valley and Ridge
The Valley and Ridge province consists of a belt of northeast-southwest trending ridges and valleys (fig. 3) formed by the differential erosion of a thick sequence of folded and faulted sedimentary rocks (Pettijohn, 1970, p.1). The availability and quality of ground water vary depending on whether flow occurs in fractures, along faults and bedding planes, in colluvium in the mountainous areas where sandstones dominate, or in solution channels in the carbonate valleys (karst areas).
Appalachian Plateau
SUSCEPTIBILITY DEsignations OF REGIONAL AQUIFERS
Making reliable estimates of ground-water ages using environmental tracers requires thorough knowledge of the hydrogeologic setting. A public supply well chosen for sampling in the VAS study is meant to represent the regional aquifer that it taps. For instance, in the layered aquifers of the Coastal Plain, a well chosen for sampling must be screened across a discrete interval in a single aquifer. Bedrock wells commonly have a long open interval, but because flow occurs in the fractures, knowledge of fracture locations controls the precision of the sampling. Therefore, only wells of known location, depth of water-bearing zones, and well construction are selected for sampling. The number and spatial distribution of wells selected will represent adequately the range of hydrogeologic conditions in each physiographic province and allow statistical comparisons among the physiographic provinces (table 2). Samples for analysis of CFCs, 3H, 3H/3He, and 14C are collected from a pumping well after stabilization of field properties (pH, specific conductance, dissolved oxygen, temperature, and turbidity.) The environmental tracers that are appropriate for the sampled aquifer system are selected for analysis (table 2). Water also is analyzed for (1) major ions to aid in the application of the geochemical model, (2) dissolved gases to estimate ground-water recharge temperature, and (3) nitrate to indicate potential near-surface sources of contamination, including fertilizers, animal wastes, and septic systems (Kroehler, 1990).
Subsequent to data collection and analysis, the susceptibility of the water supply to near-surface contamination will be evaluated. The presence of CFCs and 3H indicates that water that was in contact with the air less than 50 years ago is being pumped from the aquifer, and characterizes a well as potentially susceptible to near-surface contamination. Conversely, the absence of CFCs or 3H indicates a ground-water apparent age of greater than 50 years and characterizes a well as relatively non-susceptible to near-surface contamination. This lower susceptibility or greater age can be confirmed with a 14C derived age.
Table 2. Distribution of sample sites and
primary methods for age determinations
|
|
|
|
|
|
Coastal Plain |
Shallow aquifer system |
20 |
CFCs and Tritium/Helium-3 |
|
Middle aquifer system |
20 |
Carbon-14 |
|
|
Deep aquifer system |
11 |
Carbon-14 |
|
|
Piedmont |
Shallow system |
20 |
CFCs and Tritium/Helium-3 |
|
Deep system |
20 |
CFCs, Tritium/Helium-3, and Carbon-14 |
|
|
Blue Ridge |
Blue Ridge system |
10 |
CFCs and Tritium/Helium-3 |
|
Valley and Ridge |
Carbonate system |
20 |
CFCs and Tritium/Helium-3 |
|
Noncarbonate system |
20 |
CFCs, Tritium/Helium-3, and Carbon-14 |
|
|
Appalachian Plateau |
Appalachian Plateau system |
20 |
CFCs, Tritium/Helium-3, and Carbon-14 |
Benefits of Study
Virginia's geologic diversity makes it ideal for conducting a ground-water dating study, as the VAS study will document the utility of dating methods in determining susceptibility to near-surface contamination in multiple aquifer types. The necessity of using a method that is applicable in many settings and the benefit of using substances that do not have to be added to the ground water as tracers combine to make ground-water dating a practical technique for determining the susceptibility of Virginia's regional aquifers to near-surface contamination.
The findings of the VAS study will be used by VDH to identify sensitive and non-sensitive public supplies. This determination will allow the State to concentrate its ground-water protection efforts on the public supplies that are most vulnerable to near-surface contamination. The data generated by the VAS study will be archived for future use in addressing water-quality issues. In accordance with the Safe Drinking Water Act Amendments of 1996, the VAS study will make a long-term contribution to Virginia’s effort to help communities provide safe public drinking water.
References
Busenberg, Eurybiades, and Plummer, L.N., 1992, Use of chlorofluorocarbons (CCl3F and CCl2F2) as hydrologic tracers and age-dating tools--The alluvium and terrace system of central Oklahoma: Water Resources Research, v. 28, no. 9, p. 2257-2283.
Molina, M., and Rowland, F.S., 1974, Stratospheric sink for chlorofluoromethanes: Chlorine atom catalyzed destruction of ozone: Nature, no. 249, p. 810-812.
Derra, S., 1990, CFCs--No easy solutions: Research and Development, v. 32, p. 54-66.
Volk, C.M., Elkins, J.W., Fahey, D.W., Dutton, Gilligan, J.M., Lowenstein, M., Podolske, J.R., Chan, K.R., and Gunson, M.R., 1997, Evaluation of source gas lifetimes from stratospheric observations: Journal of Geophysical Research - Atmosphere 102 (D21), 25543-25564.
Cook, P.G., Solomon, D.K., Plummer, L.N., Busenberg, E., Schiff, S.L., 1995, Chlorofluorocarbons as tracers of groundwater transport processes in a shallow, silty sand aquifer: Water Resources Research, v. 31, no. 3, p. 425-434.
International Atomic Energy Agency (IAEA), 1981, Statistical treatment of environmental isotope data in precipitation: Vienna, IAEA, Technical Report Series No. 206.
Clark, I.D., and Fritz, Peter, 1997, Environmental isotopes in hydrogeology: New York, Lewis Publishers, 328 p.
Schlosser, Peter, 1992, Tritium/3He dating of waters in natural systems, in Isotopes of noble gases as tracers in environmental studies: Vienna, IAEA, p. 123-145.
Bradley, R.S., 1985, Quaternary paleoclimatology: Boston, Unwin Hyman, 472 p.
Coplen, T.B., 1993, Uses of environmental isotopes, in W.M. Alley, ed., Regional ground-water quality: New York, Van Nostrand Reinhold, p. 227-254.
Plummer, L.N., Parkhurst, D.L., and Thirstiness, D.C., 1983, Development of reaction models for ground-water systems: Geochimica et Cosmochimica Acta, v. 47, p. 665-686.
Plummer, L.N., Prestemon, E.C., and Parkhurst, D.L., 1991, An interactive code (NETPATH) for modeling net geochemical reactions along a flow path: 91-4078, 227 p.
______1994, An interactive code (NETPATH) for modeling net geochemical reactions along a flow path, version 2.0: 94-4169, 130 p.
Winter, T.C., Harvey, J.W., Franke, O.L., Alley, W.M., 1998, Ground water and surface water, a single resource: U.S. Geological Survey Circular 1139, 79 p.
Meng, A.A. III, and Harsh, J.F., 1988, Hydrogeologic Framework of the Virginia Coastal Plain: U.S. Geological Survey Professional Paper 1404-C.
Trapp, H., Jr., and Horn, M.A., 1997, Ground-water atlas of the United States--segment 11, Delaware, Maryland, New Jersey, North Carolina, Pennsylvania, Virginia, West Virginia: U.S. Geological Survey Hydrologic Investigations Atlas 730-L, 24 p.
Nelms, D.L., Harlow, G.E., Jr., and Hayes, D.C., 1997, Base-flow characteristics of streams in the Valley and Ridge, Blue Ridge, and Piedmont Physiographic Provinces of Virginia: U.S. Geological Survey Water Supply Paper 2457, 48 p.
Powell, J.D. and Abe, J.M., 1985, Availability and quality of ground water in the Piedmont province of Virginia: U.S. Geological Survey Water Resources Investigations Report 85-4235, 33 p.
Nelms, D.L., and Brockman, A.R., 1997, Hydrogeology of, quality and recharge ages of ground water in, Prince William County, Virginia, 1990-91: U.S. Geological Survey Water-Resources Investigations Report 97-4009, p. 58.
Smith, P.J. and Ellison, P.R., 1985, Groundwater map of Virginia: Virginia Water Control Board, Ground Water Program, Information Bulletin 560
Pettijohn, F.J., 1970, The Valley and Ridge--Stratigraphy and sedimentation, introduction, in Fisher, G.W., Pettijohn, F.J., Reed, J.C., Jr., and Weaver, K.N., eds., Studies of Appalachian geology: central and southern: New York, Wiley-Interscience, p. 1-3.
Harlow, G.E., Jr., and LeCain, G.D., 1993, Hydraulic characteristics of, and ground-water flow in, coal-bearing rocks of southwestern Virginia: U.S. Geological Survey Water Supply Paper 2388, 36 p.
Kroehler, C.J., 1990, What Do the Standards Mean? A Citizens Guide to Drinking Water Contaminants: Virginia Water Resources Research Center: Virginia Polytechnic Institute and State University, Blacksburg, 88 p.