

Sulfur hexafluoride (SF6)
is a nontoxic and extremely stable gas that is found in trace amounts in the
atmosphere. SF6 is primarily used as an electrical insulator in
high-voltage switches and industrial production has steadily increased since
1953 (Maiss and Brenninkmeijer, 1998). 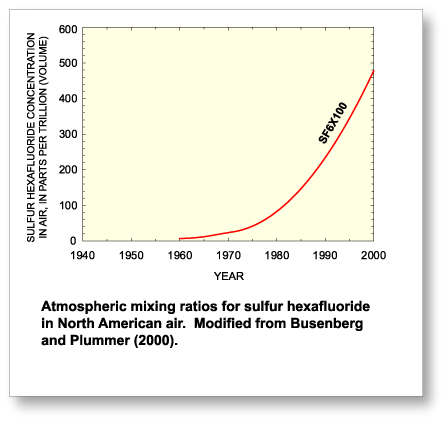 Concentrations of SF6 in the atmosphere have rapidly
increased over the past 35 years and are expected to continue to increase in the
future whereas concentrations of CFCs are expected to decline.
Current atmospheric concentrations of SF6
are between 4 and 5 pptv (parts per trillion
by volume). Busenberg and Plummer (2000) describe a method for using SF6
to date young
ground waters that is based on the known
atmospheric history and the high rate of increase in the atmosphere (7% per
year).
Concentrations of SF6 in the atmosphere have rapidly
increased over the past 35 years and are expected to continue to increase in the
future whereas concentrations of CFCs are expected to decline.
Current atmospheric concentrations of SF6
are between 4 and 5 pptv (parts per trillion
by volume). Busenberg and Plummer (2000) describe a method for using SF6
to date young
ground waters that is based on the known
atmospheric history and the high rate of increase in the atmosphere (7% per
year).
Biodegradation, sorbtion onto organic matter, and highly reducing conditions
apparently do not significantly affect SF6
concentrations in
ground water. SF6,
unlike CFCs, is
not present in common household products and contamination is not expected in
urban environments. Possible limitations of the SF6
dating method are the
presence of natural SF6
sources (minerals and igneous rocks) and addition of
atmospheric concentrations as excess air during recharge (Busenberg and Plummer
(1997).
Busenberg, Eurybiades, and Plummer, L.N., 1997, Use of sulfur hexafluoride as a dating tool and as a tracer of igneous and volcanic fluids in ground water. Geological Society of America, Salt Lake City, 1997, Abstracts and Programs, v. 29, no. 6, p. A-78.
Busenberg Eurybiades, and Plummer, L.N., 2000, Dating young ground water with sulfur hexafluoride: Natural and anthropogenic sources of sulfur hexafluoride: Water Resources Research, v. 36, no. 10, p. 3011-3030.
Maiss M., and Brenninkmeijer, C. A. M., 1998, Atmospheric SF6: Trends, sources, and prospects: Environ. Sci. Technol., v. 32, p. 3077-3086.
Plummer, L.N., and Busenberg, Eurybiades, 2000, Chlorofluorocarbons, in Cook, P.G., and Herczeg, Andrew, eds., Environmental tracers in subsurface hydrology: Kluwer Academic Publishers, p. 441-478.

